A Message from Dr. Trieu, GRMDC Medical Director
At Greater Roslindale Medical and Dental Center, our priorities are to help you stay healthy and deliver equitable, compassionate, comprehensive care to our patients, especially during the COVID-19 pandemic. We know and see the challenges our patients, staff, family and friends have faced and want you to know we are here for you. We see hope with the COVID-19 vaccine that life may return to "normal" again.
As always, we want you to make an informed decision that is right for you based on trusted information. Please reach out to your healthcare team if you have questions or would like to discuss the COVID vaccine. Many GRMDC staff are vaccinated because we want to protect our patients and families.
There is a lot of information out there. We recommend relying on local and state public health departments and the CDC for accurate information. GRMDC is ablfrae to vaccinate anyone interested in receiving the vaccine - just ask us!
From all of us at GRMDC, we hope you and your family continue to stay healthy.
Masking: With the hopeful increase of COVID vaccinated people in our local communities, the promise of unmasking and living life pre-pandemic style is tempting. I would first encourage all unvaccinated individuals to continue masking to protect your family members, especially children. We know that masking and distancing can prevent the spread of COVID. For vaccinated people, the CDC is allowing returning to pre-pandemic life in most cases. This is a giant change for us - masks have become a part of our wardrobe! Consider continuing to mask if you worry about the health of a close unvaccinated family member. We should all remember to follow masking guidelines set forth by the public/private entities we participate in including public transportation, businesses, restaurants. You may or may not be ready to unmask and that's ok. When and if masks come off for you, know that the vaccine is effective at preventing serious illness from COVID.
TThe Moderna & Pfizer vaccines are mRNA vaccines. mRNA is not the same as DNA. mRNA works by telling our bodies to make a protein that then produces antibodies to the virus. These antibodies help protect you from the virus that causes COVID-19. The Johnson & Johnson (J&J) vaccine is a viral vector vaccine. A viral vector is a virus that has been altered, so it doesn't cause illness but still makes your immune system build up specific defenses.
The trial results for the Pfizer and Moderna vaccines showed >94% efficacy at preventing COVID-19. J & J has an 86% efficacy rate in worldwide tests. By getting vaccinated, you reduce your risk of disease, hospitalization, severe complications, and even death. Right now, the risk of contracting COVID-19 in Massachusetts is high. In addition to mask-wearing, physical distancing, and handwashing, this is one tool that will help reduce your risk of illness, be hospitalized, and prevent the health care system from being further overwhelmed.
The available COVID-19 vaccines are highly effective and safe and have been through a strict clinical review. All three vaccines have been shown in clinical trials to be 100 percent effective against hospitalization and death related to COVID-19. You will not be able to choose which you receive; which one you get will depend on our supply of each at the time. The best vaccine to get is the one that is immediately available to you.
COVID-19 vaccines are very effective in preventing COVID-19. While they helped prevent COVID-19 generally, they were particularly good at preventing severe cases of the disease. And in clinical trials, they all were 100 percent effective in preventing hospitalization and death related to COVID-19.
However, it’s important to keep wearing a mask and distancing because:
- Not everyone will get the vaccine at once. Following public health guidelines will help protect anyone who hasn’t gotten the vaccine yet.
- Although it’s not likely, it’s still possible to get COVID-19 after getting the vaccine, as no vaccine is 100 percent effective.
- We’re not sure yet how long the vaccine will protect you from getting COVID-19.
No. The cells that use the mRNA vaccine get rid of the mRNA after they finish using it, and every component of the vaccine will be gone shortly after. The mRNA never gets into the part of the cell where your body’s DNA is located. The J&J vaccine also does not enter your body’s DNA.
Yes. COVID-19 vaccines have gone through the same trials as other approved vaccines. The COVID-19 vaccines have met the high safety standards these trials set. The clinical trial process for COVID-19 vaccines was much quicker than for other vaccines, but it was done just as carefully. Steps were not skipped, but instead, given the urgent need, different phases in the trial were run simultaneously rather than one after the after. More than 70,000 people took the other COVID-19 vaccines as part of clinical trials, which is a large number of people. The current recommendation is vaccination of 18 and older; more information to come for the pediatric population.
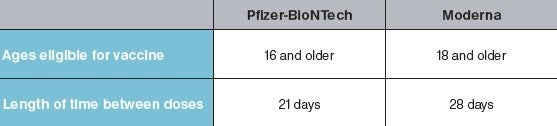
Current recommendation is vaccination of 18 and older, more information to come for the pediatric population.
TThe vaccine will be given as a shot in the upper arm. The first vaccines that will be available require two doses. For the Pfizer vaccine, the second shot will be three weeks after the first. For the Moderna vaccine, it will be four weeks later. You must get both shots. If you don’t, you won’t be as well-protected from COVID-19 as you could be. Recipients should get the second dose from the same manufacturer as their first dose. Only one dose is needed for the Johnson & Johnson vaccine.
We don’t know how long protection will last. The studies that are going on now will help to answer that question.
The goal is for everyone who wants it to eventually get the COVID-19 vaccine. Because of this, the vaccine will be given in phases. When you get the vaccine will be based on things like your job, your age, and your health. The timeline depends on factors such as: how many vaccines are approved, how many vaccines each company is able to make, how many people get vaccinated in earlier groups. GRMDC plans to outreach to patients eligible for vaccination via website, letters and MyChart.
At this time, GRMDC is vaccinating patients by phases; please check to see which phase you will be eligible for. We will send information in multiple ways to inform our patients (you) of how to get the vaccine.
The federal government has committed to providing the vaccine at no cost to all individuals who want the vaccine. In Massachusetts, insurance companies and providers have agreed to provide the vaccine without out-of-pocket fees or co-payments.
No. It is not currently mandatory to get the vaccine. The state or other employers could eventually make getting the vaccine required for certain activities, such as going back to work or going to public school – much like other vaccines are. However, we don’t know of any current plans to make COVID-19 vaccines mandatory, and the Biden administration has said that they do not plan to make the vaccine mandatory throughout the U.S.
While we know the COVID-19 vaccines can prevent severe COVID-19 infections, we do not yet know how effective the vaccines are in preventing asymptomatic infection, which is when you are infected with COVID-19 but don’t have any symptoms. It may be possible to still spread COVID-19 after getting a vaccine, so it is still important to wear masks and keep distances between people. As people start to get the vaccines, researchers will be looking at how well they prevent asymptomatic infection.
The goal is for everyone to be able to easily get a COVID-19 vaccine as soon as large quantities are available. That is why, early in the response, thefederal government began investing in select vaccine manufacturers to help them increase their ability to quickly make and distribute a large amount of COVID-19 vaccine. This will allow the U.S. to start with as much vaccine as possible and continually increase the supply in the weeks and months to follow. Several thousand vaccination providers will be available, including doctors’ offices, retail pharmacies, hospitals, and federally qualified health centers.
Yes. While experts learn more about the protection that COVID-19 vaccines provide under real-life conditions, it will be important for everyone to continue using all the tools available to us to help stop this pandemic, like covering your mouth and nose with a mask, washing hands often, and staying at least 6 feet away from others. Together, COVID-19 vaccination and following CDC’s recommendations for how to protect yourself and others will offer the best protection from getting and spreading COVID-19. Experts need to understand more about the protection that COVID-19 vaccines provide before deciding to change recommendations on steps everyone should take to slow the spread of the virus that causes COVID-19. Other factors, including how many people get vaccinated and how the virus is spreading in communities, will also affect this decision. (source: CDC.gov Frequently Asked Questions about COVID-19 Vaccination)
There is not enough information currently available to say if or when CDC will stop recommending that people wear masks and avoiding close contact with others to help prevent the spread of the virus that causes COVID-19. Experts need to understand more about the protection that COVID-19 vaccines provide before making that decision. Other factors, including how many people get vaccinated and how the virus is spreading in communities, will also affect this decision. (source: CDC.gov Frequently Asked Questions about COVID-19 Vaccination)
An Emergency Use Authorization (EUA) is a mechanism to facilitate the availability and use of medical countermeasures, including vaccines, during public health emergencies, such as the current COVID-19 pandemic. The Pfizer, Moderna & J&J vaccines were approved under emergency authorization because of how serious the pandemic is. It's likely that other vaccines will be approved this way. But the safety standards for emergency authorization are close to the same as the ones vaccines have to meet for regular authorization. Expert groups will also keep looking at the COVID-19 vaccine’s safety after people start to take it.
Some people in the clinical trials did report side effects. Side effects have generally been mild, and are a sign the immune system is working, there have been some severe adverse effects occur, but they have been rare. Reported side effects include headaches, fatigue, chills, and soreness at the injection site. A small number of participants had a fever. For some people, these side effects were worse after the second dose. Side effects from a vaccine usually go away on their own within a few days. If your side effects last more than 48 hours, speak to your doctor. There have been no serious safety concerns noted in the studies of these vaccines.
CDC is working with partners across the country to make sure people have the information they need to be confident in deciding to get vaccinated. Key priorities for CDC are:
- Regularly sharing clear and accurate information with people to make sure they understand the risks and benefits of getting vaccinated and can make informed decisions.
- Helping healthcare personnel feel confident in their decision to get a COVID-19 vaccine and helping healthcare providers answer their patients’ questions about the vaccine.
Engaging communities and individuals in an equitable and inclusive way to ensure that people have opportunities to ask questions and get clear, accurate information about the COVID-19 vaccine.
Easy access to COVID-19 vaccines is equally critical. CDC is working with public health, healthcare providers, and other partners to make sure people can easily get a COVID-19 vaccine and without cost.
Yes. Experts recommend getting the vaccine even if you already had COVID-19. You can receive the vaccine as soon as you are released from isolation and/or quarantine. It is unlikely a person will be infected with COVID-19 within 90 days of your infection so it is also reasonable to wait 3 months after your infection.
If you received monoclonal antibody treatment for COVID-19, you will need to wait 90 days prior to getting your COVID-19 vaccination.
The FDA recommends that people who have severe allergies to any ingredient in the Pfizer-BioNTech or Moderna vaccine do not get this vaccine. In addition, they recommend that you should not get the second dose if you have a severe allergic reaction to the first dose. Everyone who gets the vaccine will be watched for 15 minutes after the injection to make sure they do not have any signs of an allergic reaction. People who have severe allergies to other vaccines or injectable medications will be watched for 30 minutes. Neither vaccine contains egg. Once you are able to get the vaccine, talk to your primary care physician or allergist if you have concerns.
In Massachusetts, Black and Latino/a communities have been affected by COVID 19 illness and deaths at much higher rates than other communities, and we recognize that our patients may know or personally have been affected by COVID 19.
The U.S. clinical trials for Moderna and Pfizer included 10% Black and 13% Hispanic/Latino/a participants. Data from the Pfizer studies showed that the vaccine has similar success rates in white, Black, and Latino/a people. In fact, the Dr. Kizzmekia Corbett, a black research scientist, was a key contributor in the development of the NIH/Moderna vaccine.
Read more about herehere.
There is no evidence that the COVID-19 vaccine affects fertility. In the safety data from the Pfizer trial, the same proportion of people got pregnant in the vaccine group as the placebo group. Based on this, the vaccine is recommended even if you are planning to get pregnant soon.
Although there is currently no data specifically on COVID-19 vaccine safety in pregnant and breastfeeding people, there is very low concern for safety issues, based on experience with other vaccines and the science of the COVID-19 vaccines. Therefore, based on guidance by the FDA and the CDC, people who are pregnant or breastfeeding may choose to have the COVID-19 vaccine.
If you are pregnant or breastfeeding, please talk to your doctor about potential risks and benefits.We have seen that pregnant people are at increased risk for severe COVID, which means that the benefits may outweigh the risks for many. As the vaccine is given to more people, researchers are looking at the benefits and potential risks for pregnant and breastfeeding people
The Pfizer-BioNTech vaccine worked as well in older adults as it did in younger adults. In this trial, about 45 % of participants were ages 56-85.
The FDA approved the Pfizer vaccine for anyone over 16 years of age. Those under age 18 are not eligible to receive the Moderna or J&J vaccine as there are no data on the safety and efficacy of this population. When they approve other vaccines, they'll give a recommended minimum age for each. A small number of children as young as 12 years of age were included in the vaccine studies. There were no safety concerns noted for this group. However, COVID-19 vaccines haven’t been studied in younger children. These studies are now being planned.
No, you will need to wait two weeks after getting the COVID-19 vaccine before getting other immunizations.
CDC and FDA encourage the public to report possible side effects (called adverse events) to the Vaccine Adverse Event Reporting System (VAERS). This national system collects these data to look for adverse events that are unexpected, appear to happen more often than expected, or have unusual patterns of occurrence. Learn about the difference between a vaccine side effect and an adverse event. Reports to VAERS help CDC monitor the safety of vaccines. Safety is a top priority.
Healthcare providers will be required to report certain adverse events following vaccination to VAERS. Healthcare providers also have to adhere to any revised safety reporting requirements according to FDA’s conditions of authorized use throughout the duration of any Emergency Use Authorization; these requirements would be posted on FDA’s website.
Yes. We do not yet have data to say for how long the COVID-19 vaccines provide protection from getting sick and whether the vaccines also keep people from spreading COVID-19. Experts need to understand more about the protection that COVID-19 vaccines provide before changes are made to public health quarantine recommendations. Other factors, like how many people get vaccinated and how the virus is spreading in communities, will also affect this decision.
Anyone who lives in Massachusetts, regardless of immigration status, can receive a COVID-19 vaccination at a site in the state. You do not need to provide an ID or Social Security number to get the COVID-19 vaccine. Getting the COVID-19 vaccine will not have any negative impact on your chances of getting a green card.
